Osmosis and Osmotic Pressure
Osmosis and Osmotic Pressure: Overview
This topic covers concepts such as isotonic solution, hypertonic solution, osmosis, semi-permeable membrane and osmotic pressure.
Important Questions on Osmosis and Osmotic Pressure
Which of the following colligative property is preferred for the determination of molar masses of solutes in solution
of molal urea solution (density at ) is mixed with of a solution containing of urea. Both the solutions were prepared in the same solvent. The osmotic pressure (in torr) of the resulting at is
[Use : Molar mass of urea ; gas constant, ;
Assume,
Which one of the following is an isotonic pair of solution?
A glucose solution is to be injected into the blood stream. It must have the same_______ as the blood stream.
Solution of of non-electrolyte (A) prepared by dissolving it in of water exerts the same osmotic pressure as that of glucose solution at the same temperature. The empirical formula of is The molecular mass of is (Nearest integer)
solution is isotonic with glucose solution. Percentage dissociation of is _____ (Nearest integer)
Consider the following pairs of solution which will be isotonic at the same temperature. The number of pairs of solutions is/ are _____
A. and urea
B. and
C. and
D. and
Find out the concentration of glucose solution in gram per litre is isotonic to sucrose solution at ?
Osmotic pressure of solution of glucose is and that of solution of cane sugar is . The osmotic pressure of the mixture containing equal volumes of the two solutions will be:
If of glucose (molar mass ) is dissolved in of water at , the osmotic pressure of the solution will be
solution of urea is isotonic with solution of a non-volatile solute of molar mass . The value of is
At , urea solution has an osmotic pressure of . On dilution by times, its osmotic pressure decreased to at . The dilution factor is approximately
A solution containing of urea is isotonic with a solution containing of a non-electrolytic solute . The molar mass of is
Assertion (A) : Blood cells collapse when suspended in saline water
Reason (R): Cell membrane dissolves in saline water
The osmotic pressure of human blood is equal to the osmotic pressure with 0.1 (N) NaCl solution. If the vant half factor of NaCl is 1.82, what
will be the osmotic pressure of the blood at 37 °C?
Round off your answer to the nearest integer
The Osmotic pressure of aqueous solution of and respectively are in the ratio
Which method is used to remove salts from sea water?
Explain why the equimolar solutions of sodium chloride and sodium sulphate are not isotonic?
Given in the figure is the sketch of a plant carrying out a process:
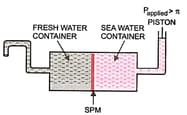
(iv) Give one practical use of plant.
Given in the figure is the sketch of a plant carrying out a process:
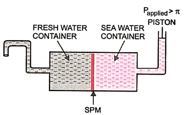
(iii) Name one SPM which can be used in this plant.
